Mass Number Of Oxygen
Mass number is an integer equal to the sum of the number of protons and neutrons of an atomic nucleus.

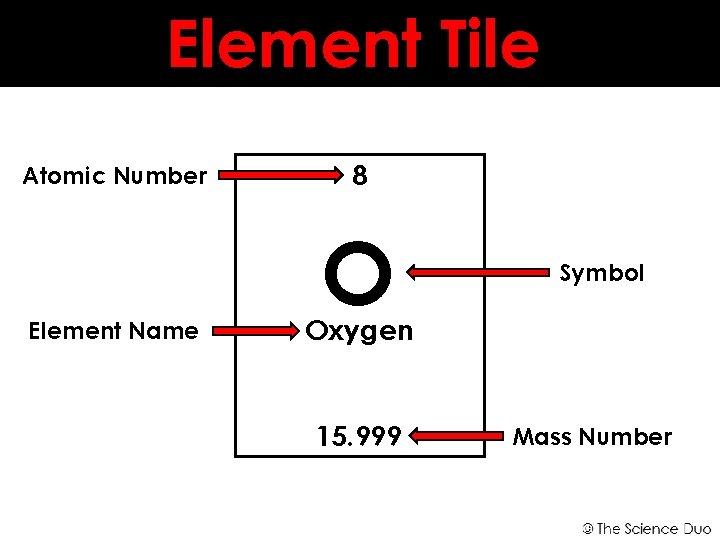
The mass number allows enable us to tell the difference between elements with a different number of neutrons, called isotopes.

Mass Number Of Oxygen And Sulphur Atom
Answer: The mass number of an oxygen isotope that has nine neutrons is 17 and isotope is called as Oxygen-17. Explanation: Atomic number is defined as the number of protons or number of electrons that are present in an atom. For a neutral atom: Atomic number = Number of electrons = Number of proton. Atomic number= Number of protons = 8. Oxygen is an element displayed by the symbol O, and atomic number 8. It is an essential element for human survival. It is an essential element for human survival. Decreased oxygen levels may be treated with medical oxygen therapy. Atomic Mass of Oxygen. Atomic mass of Oxygen is 15.9994 u. The atomic mass is the mass of an atom. The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain specific isotope of an element.
- Oxygen accounts for about 23% of the atmosphere's mass with pairs of oxygen atoms stuck together to make dioxygen molecules, but it's not just in the air, we breathe. Overall, it's the most abundant element on the earth's surface and the third most abundant in the universe after hydrogen and helium.
- Answer (1 of 4): Oxygen has 8 neutrons and 8 protons in its nucleus which tells us that the mass number of oxygen as 16.
The mass number is a count and is unitless.
Neutron Number Of Oxygen
Formula to calculate mass number.
Example 1:
The hydrogen atom doesn’t have a neutron but it has 1 proton, so the mass number of hydrogen is 1.
Mass Number Of Oxygen 16
Example 2:
Oxygen Atomic Mass And Number
Oxygen atom has 8 protons and 8 neutrons. Determine the mass number.
Atomic Mass Number Of Oxygen

Thus, oxygen atom mass number is 16.
